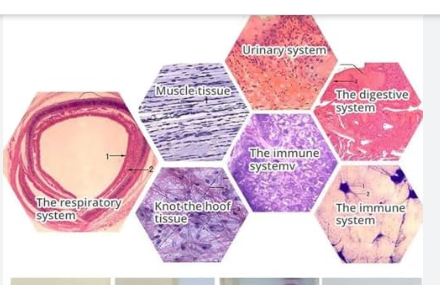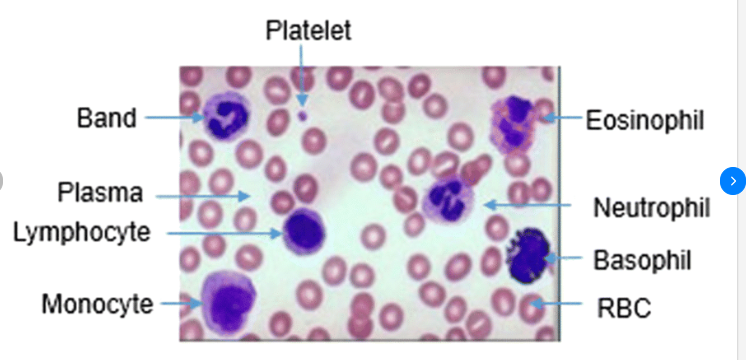
This course provides a thorough understanding of Good Clinical Laboratory Practices (GCLP), which are essential for ensuring the quality and reliability of laboratory results in clinical research. It is designed for laboratory technicians, researchers, and healthcare professionals involved in clinical trials and laboratory management. Participants will learn about regulatory requirements, quality management systems, standard operating procedures (SOPs), and ethical considerations necessary for maintaining high standards in clinical laboratories.
- Teacher: Nancy Adipo
- Teacher: Richard Yahuma